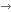University of Szeged
Albert Szent-Györgyi Medical School
Foreign Students' Secretariat
Your Education. Our Mission.
Organoids for the future of health care at the University of Szeged
The SZTE IKIKK (Centre of Excellence for Interdisciplinary Research, Development and Innovation) Organoid Facility, newly established at the University of Szeged, will offer a unique opportunity for researchers, pharmaceutical companies and clinical partners: researchers will be able to use organoid models to study disease mechanisms, test the effects of drugs and develop personalized therapies.
We spoke with Dr. József Maléth, professional manager of the SZTE IKIKK Competence Center of Translational Biomedicine, about the Organoid Facility.
- What is an ’organoid’ and why is it important in research?
An organoid is a miniature, three-dimensional cell culture that can mimic the structure and function of an organ or tissue, such as the intestinal tract, pancreas, or lungs. We produce them from human cells, so-called primary cells, which are cells that come directly from patients. This is a huge step forward, as the animal models and two-dimensional cell cultures used previously could only reproduce the actual functioning of the human body to a limited extent. Organoids, on the other hand, are made from real human cells, so they mimic human tissues and diseases much better, which means they can shorten development processes.
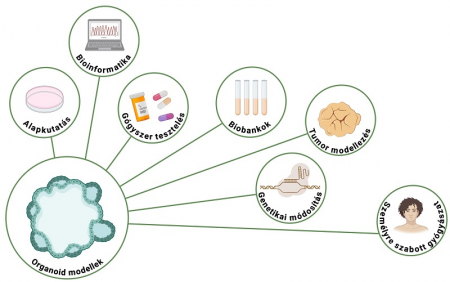
Organoids now play a key role in translational biomedicine, i.e., the field of research that brings laboratory results to the bedside. These models not only provide a more accurate picture of how diseases develop, but also help us understand how drugs work and what side effects can be expected. The models also enable us to study the mechanisms of cancerous or inflammatory diseases directly on human samples, test drugs, and even develop patient-specific therapies.
We have been working with organoid models at the University of Szeged for many years, and over the years we have accumulated a wealth of experience, methods and infrastructure in this field. Now the time has come to concentrate this knowledge in a joint, well-organized, dedicated center—and that is how the idea for the Organoid Facility was born.
- Why did you opt for a separate organoid center at SZTE?
The Organoid Facility was established as a professional center that bridges the growing demand for organoid models derived from human cells and research and industrial capacities. We not only provide the expertise needed to produce cell cultures, but also the laboratory background and technological infrastructure – and we do this by creating a high-quality, standardized environment.
The Organoid Facility aims to serve the needs of a variety of partners. We offer academic researchers the opportunity to study developmental processes, disease progression and therapeutic responses using state-of-the-art organoid models. This could lead to new research directions, potentially even patents. For pharmaceutical and biotechnology companies, we offer a platform where they can effectively test the effects and possible side effects of their new compounds. This can significantly shorten development time and reduce costs. For clinical centers and hospitals, we provide the opportunity to test potential treatment strategies on patient-specific organoids. In the longer term, this could contribute to the development of precision oncology and enable patients to receive truly personalized therapies.

- How does the SZTE IKIKK Organoid Facility differ from other similar facilities abroad?
One of the greatest strengths of the SZTE IKIKK Organoid Facility—which is rare even at an international level—is that it does not rely exclusively on laboratory-generated stem cell models (such as iPSC-based organoids), but works with real, clinically relevant human tissues. This means that our models are closer to the functioning of the real human body, thus providing more reliable results, both in basic research and drug development.
What's more, we don't just work with one type of tissue. Our facility is based on three main types of samples: healthy, cancerous (including metastatic), and inflamed tissues. From these, we isolate not only organoids, but also other cell types—such as stromal cells or primary cells—which we can then combine in a project-specific manner. This also allows us to create complex co-cultures, where the tissue environment can be modelled more realistically.