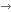Microcirculation Laboratory
Head: Dr. Andrea Szabó
Main Topics:
Examination and modulation of the microcirculatory the consequences of different diseases related to surgical procedures under clinical or experimental conditions. Examination of the microcirculatory effects of ischemia/reperfusion and sepsis by assessing (1) capillary perfusion, (2) local and systemic inflammatory processes and (3) integrity of endothelial glycocalyx. Therapeutic possibilities to modulate microcirculatory reactions.
Researchers: Almost every coworker of the institute routinely uses devices for examination of the microcirculation. Gabriella Varga and Dániel Érces use these methods during their international cooperation, but Marietta Poles also uses them on a daily basis. Our important clinical partners: Ágnes Janovszky (Department of Oral And Maxillofacial Surgery, University of Szeged), Ildikó László and Nándor Öveges (Department OF Anaesthesiology and Intensive Therapy, University of Szeged).
Hypoxia-reoxygenation injury and sepsis are among the most important research interests of the Microcirculation Laboratory. Activity of this laboratory brings the interests of many clinical areas together (urology, cardiac surgery, orthopedics, ophthalmology, gastroenterology, etc.).
Detection and quantification of the extent of microcirculatory insufficiency also represent important therapeutic targets. The fluorescence intravital video microscope (IVM) can be used only in animal models at present, but it is an excellent tool not only for visualization of intravascular cellular reactions, but also to assess changes in microvascular vascular diameter, flow rate and vascular permeability. After ex vivo or in vivostaining of cellular components of the blood (e.g., Rhodamine 6G is used to label leukocytes), or the plasma (e.g. fluorescein isothiocyanate dextran), microcirculatory changes within living tissue can continuously be observed and recorded using a video camera. Evaluation is performed via off-line analysis of the recorded images. The method is suitable for in situ direct observation of the microcirculation of most organs, including e.g. the meninges, small intestine, urinary bladder, liver, pancreas, lungs, muscle or the periosteum (at a tissue depth of about 200 micrometers).
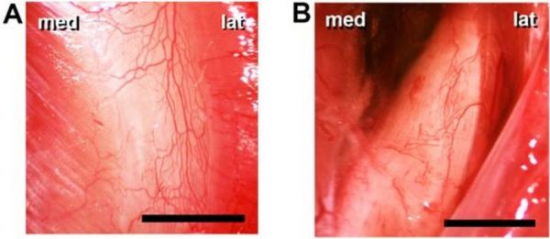
Figure 1. Examples of tissue preparations used for IVM. Operating microscopic view of the left anteromedial tibial periosteum (A) and the right mandibular periosteum (B) in Sprague-Dawley rats. Medial and lateral sides are indicated (med, lat). The bar denotes 1000 µm.
With the IVM technique, distinct steps and parameters of microcirculatory inflammatory reactions can numerically expressed (tethering of white blood cells, their gradual attachment e.g. rolling and adhesion to the endothelium of the postcapillary venules, as well as their transmigration to the interstitium and their tissue accumulation). Spatial resolution can be further improved by confocal microscopy, one of which is the so-called laser scanning confocal endomicroscopy (LSCM); this is also available at our institute. OptiScan is a LSCM-based fluorescent endomicroscopic system that is suitable for morphological analysis in vivo and in situ at 100 to 200 micrometers in depth, without any histological embedding.
Intravital microscopic image of the microcirculation of the periosteum (red blood cell staining with fluorescein isothiocyanate) in a rat.
Fluorescent IVM imaging provides an excellent opportunity for direct observation of the microcirculation under experimental conditions, but their clinical use is still limited. Their obstacles are mainly derived from the transillumination approach, when fluorescence dyes are given for contrast enhancement plus the size of the devices is usually also problematic for a clinical use. Most of these drawbacks are partially eliminated by the OPS (Orthogonal Polarization Spectral) imaging and the so-called "Incident dark field" (IDF) techniques. The OPS technique illuminates the examined object with linearly polarized light, where, imaging is done by the specially filtered light reflected from the tissues which outlines the hemoglobin-containing structures, such as the microvessels, at a tissue depth of about 200-300 mm. Thus, no contrast agent is needed for this method. A newer version of the method is the "incident dark field" (IDF) technique, which already has enhanced resolution and automatic data analysis mode. Due to their non-invasive nature, the latter two methods have proven to be useful in determining the prognosis and characterizing the efficacy of the therapeutic intervention by analysing the microcirculation of the oral cavity of patients in the Intensive Care Unit, even in critical intensive care patients. In addition to its clinical use, under experimental conditions usage of the IVM also can be very informative about quantifying the circulatory efficacy of the superficial tissues of the organs (listed above).
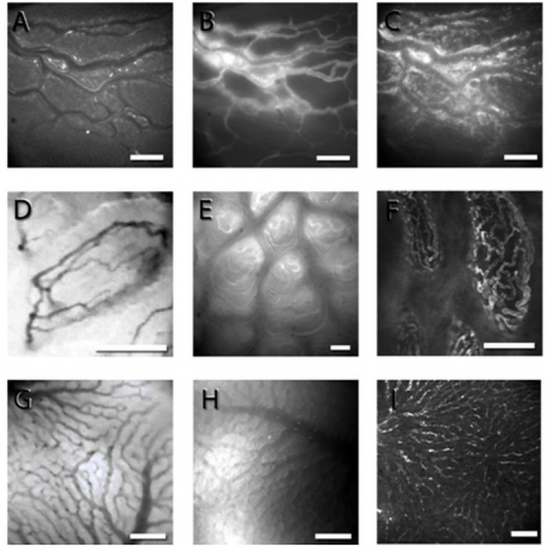
Figure 2.Representative micrographs showing the intestine and the liver with conventional fluorescence IVM (A-C, E, H), OPS (D, G) and laser scanning confocal microscopy (F, I). FITC-labeled red blood cells (A), plasma labeling (B) and rhodamine 6G-labeled leukocytes in the longitudinal muscle layer of the rat ileum (C); OPS images of the intestinal villi (D) and liver hepatic sinusoids (G) in Sprague-Dawley rats. FITC labeling of the microvessels in the intestinal villi in BALB/c mice as detected with fluorescence IVM (E) and in the intestinal villi (F) and the liver (I) in rats using laser scanning confocal microscopy. Bar denotes 100 µm.
The IVM images were taken by using a Zeiss Axiotech Vario 100HD IVM microscope, 100W HBO mercury lamp, Acroplan 20× water immersion objective, Carl Zeiss GmbH, Jena, Germany) and a CCD camera (Teli CS8320Bi, Toshiba Teli Corporation, Osaka, Japan). The OPS images were taken by the Cytoscan A/R device (Cytometrics, Philadelphia, PA, USA).
The next non-invasive technique is the laser Doppler flowmetry of which measurement depth is 0.5–1 mm in volume of approximately 1 mm3 in the skin. The methodological background of laser Doppler monitoring is the “Doppler shift” phenomenon. When a monochromatic laser light enters the tissues and meets moving blood cells, it undergoes changes in wavelength (in amplitude and frequency). The Doppler technique provides “perfusion units” which cannot directly be converted to flow (e.g., ml/min), but rather a flux unit without dimension.
The method is primarily used in the clinic to study the microcirculation of the skin, and it can be used to quantify chronic, primarily endothelium-dependent circulatory disorders through various provocation tests (eg, clamping / thawing, hot-evoked flow escalation). It also can be used for the examination of skin, mucous membranes and abdominal organs under experimental conditions.
Table 1. Brief overview of the available methodology for the detection of functional
|
Method |
Species/tissue |
Parameters |
Indication |
Reference |
|
Conventional fluorescence intravital microscopy (IVM) |
animal models/superficial tissue layers |
FCD, red blood cell velocity, blood flow, microvascular permeability, PMN-endothelial interactions |
ischemia-reperfusion, shock (hypovolemic, septic) wound healing, tumors |
Link to our full text publication using fluorescence intravital microscopy |
|
Confocal Microscopy, Laser Scanning Confocal Microscopy (LSCM) |
animal models/superficial tissue layers |
(morphology), microvascular permeability, subcellular events |
ischemia-reperfusion, shock (hypovolemic, septic) wound healing, tumors |
Link to our full text publication using laser scanning confocal microscopy |
|
“Orthogonal polarization spectral imaging” (OPS), and “incident dark-field” (IDF) technique |
human and animal models/superficial tissue layers |
vascular density,
vessel diameter, red blood cell velocity,
heterogeneity of perfusion (microvascular blood flow)
|
ischemia-reperfusion, shock (hypovolemic, septic) wound healing, tumors . |
Link to our full text publication using OPS |
|
Laser Doppler perfusion monitoring |
human and animal models/superficial tissue layers |
flux, velocity and
concentration of the moving blood cells
|
human: skin animals: perfusion deficit situations (ischemia-reperfusion, shock (hypovolemic, septic) wound healing, tumors). |
Link to our full text publication using laser Doppler flowmetry or the O2C technique |
characteristics of the microvasculature at our institute
The most important experimental instruments available at our Institute are summarized in the following table:
Link to our summary publications on microcirculation.
List of main instruments and to papers published by the Microcirculation Laboratory
• Zeiss Axiotech Vario 100HD Fluorescent Intravital Microscope (IVM) (100 W HBO with Acroplan 5-20-63x Water Immersion Lens)
• OptiScan Five1 (OptiScan Ltd, Mulgrave, Australia)
• Cytoscan A / R device (Cytometrics, PA, USA) (OPS system)
• Cytocam (Braedius Medical, Huizen, The Netherlands) (IDF system)
• Laser Doppler Flowmeter (Perimed AB, Stockholm, Sweden)
Email: office.expsur@med.u-szeged.hu
Head of Department: Prof. Dr. Boros Mihály